Methanol via Methane (CH4) Catalytic Decomposition, and CO2
1. Introduction
Although the traditional paths to making methanol require gasification of natural gas and then catalytic methanol formation from the resulting syngas (H2 and CO), there are several catalytic paths to creating methanol that don't require the gasification step. One involves decomposing methane (CH4) into H2 and C, and then combining CO2 with H2 to make methanol. We will focus on this herein.
2. Methanol from H2 and CO2
Methanol can be made from H2 and CO2 using a catalyst. The H2 is often considered obtainable from electrolytic decomposition of water, and CO2 from power plant effluent and the like or directly from the air. We examine this approach later, but next we consider another approach which uses natural gas and CO2.
2.1. Direct decomposition of natural gas (methane) into H2 and C
Thermal Ddecomposition - TD - (or pyrolysis) of methane into its constituent elements is considered an environmentally attractive approach to the production of hydrogen from natural gas (NG). The process produces no CO/CO2 by-products, which eliminates the need for water gas shift and CO2 removal stages required by conventional hydrogen production processes, e.g., steam methane reforming (SMR), coal gasification, etc. This significantly simplifies the process and dramatically reduces overall CO2 emissions.
Thermal Decomposition (TD), which is sometimes called Thermal Catalytic Decomposition (TCD), of NG is a well-known process. It has long been used for the production of carbon black with hydrogen being used as a supplemental gaseous fuel. The process was practiced batchwise using firebrick contact and required elevated temperatures (up to 1500 °C). The energy costs are obviously large,and the resulting CO2 emissions are also large.
Because the thermal decomposition of methane requires elevated temperatures (in excess of 1100 °C ), much research on the development of efficient catalysts for the process, that would not require bringing the methane to such high temperatures, has been conducted since early 1960s.
The figure below from Muradov et al [14] summarizes the catalysts story.
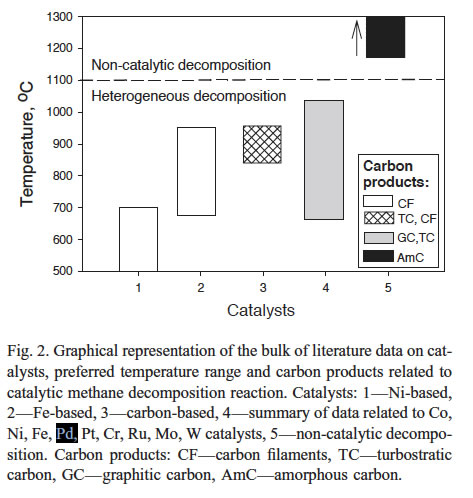
Most of the efforts in this area were focused on methane decomposition over transition metal catalysts (e.g., Ni, Fe, Co, etc.) (e.g., [4–6]). These studies point to a catalyst deactivation problem associated with the carbon being deposited on the catalyst's surface; in most cases, carbon was burned off of the catalyst's surface in order to regenerate its original activity (e.g., [4]). Combustion of carbon deposits, however, is not an environmentally friendly solution, since it results in production of large amounts of CO2 byproduct (comparable with that from Steam Methane Reforming - SMR - process). Thus, classical TCD was not a preferred technique.
Later, the technical feasibility of using carbons as catalysts for methane decomposition reaction was demonstrated in [7–9]. The use of carbon-based catalysts offers certain advantages over metal catalysts: (1) low cost, (2) high temperature resistance, (3) tolerance to sulfur and other potentially harmful impurities in the feedstock, (4) production of a marketable byproduct -- carbon (that could substantially reduce the net cost of hydrogen production), and (5) effect to mitigate overall CO2 emissions from the process. The data on the catalytic activity and stability of carbon materials of different origin and structure including a wide range of activated carbons (AC), carbon blacks (CB), graphites, nanostructured carbons, etc., toward methane decomposition reaction was reported in [8]. The data indicated that amorphous forms of carbon, e.g., CB and AC have significantly higher catalytic activity toward methane decomposition at moderate temperatures (800–900°C) compared to structurally ordered carbons (e.g., graphite). Factors governing catalytic activity of carbons and the nature of active sites are discussed in [10].
Without a catalysts, control experiments using an inert contact (silica gel) demonstrated that no appreciable thermal (i.e., non-catalytic) decomposition of methane occurs at 850-900°C (temperatures must be greater than ~ 1200°C).
The catalytic decomposition of methane is an endothermic reaction; the heat input requirements for the process at 850 and 900°C are:
CH4 → C + 2H2, delta(H) = 89, 750 J/mol (at 850°C),
delta(H) = 89, 989 J/mol (at 900°C). (4)
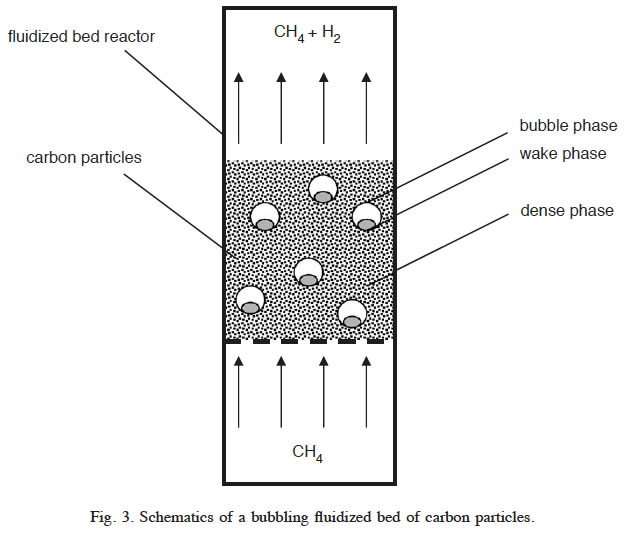
Initially, the temperatures of methane stream and carbon particles entering the FBR are assumed to be 500 and 900°C, respectively. The process heat input is provided by the incoming carbon particles. Thermal energy is also required to heat up the incoming methane stream from 500°C to the reactor bed temperature. A preheated methane stream (144.4 mol/s) at 500°C enters the FBR containing catalytically active carbon particles, where methane decomposes at the temperature of 850–900°C and a pressure of 200 kPa. Based on the experimental results of AC-catalyzed methane decomposition in a small-scale FBR, average methane conversion is assumed to be 38%.
In all cases, carbon produced was burned off the catalyst surface to regenerate its initial activity. In this regard, these processes display no significant advantages over the conventional processes (for example, SR) because of large CO2 emissions.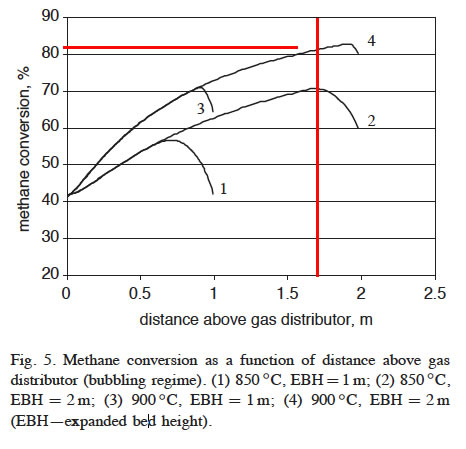
Top
2.1.1. Catalytic deactivation issues and resolutions
Although use of a catalyst can enable the reaction to occur at lower temperatures than the 1200◦C otherwise required, the catalysts looses its reactivity over time. The kinetic curves of methane decomposition over AC and CB show an initial drop in the catalytic activity followed by a relatively mild decline in methane decomposition rate. It was demonstrated [13] that carbon catalyst deactivation could mainly be attributed to the loss in the catalyst surface area due to carbon deposition.
In [13] it was demonstrated that catalytic activity of carbon catalysts could be completely recovered after their treatment with activating agents (steam, CO2 or their mixtures). Fig. 1 illustrates the results of the experiment where carbon catalyst deactivation was followed by the regeneration of its catalytic activity. In this experiment, a fresh activated carbon (AC-lignite) catalyst with the surface area of 670m2/g was exposed to methane at 850°C for 1 h resulting in the decrease in carbon surface area (to 324m2/g) and, consequently, partial deactivation of the catalyst. This was followed by steam treatment of the catalyst at 950°C for 0.5 h, which caused the increase in carbon surface area to 617m2/g and, as a result, almost complete restoration of its original catalytic activity toward methane decomposition reaction. The procedure was repeated several times, and every time we observed complete regeneration of carbon catalytic activity after the steam activation procedure. The above experiments demonstrate that it would be technically feasible to arrange a continuous process for simultaneous production of both hydrogen and carbon via Thermocatalytic decomposition (TCD) of NG.
Average methane conversions in the Fluidized Bed Reactor (FBR) (at 850–900°C) during first 0.5 h were measured at the range of 30–45% (depending on the type of AC used), which corresponds to an average hydrogen concentration of approximately 50 vol% in the effluent gas balanced with unconverted methane and other minor species.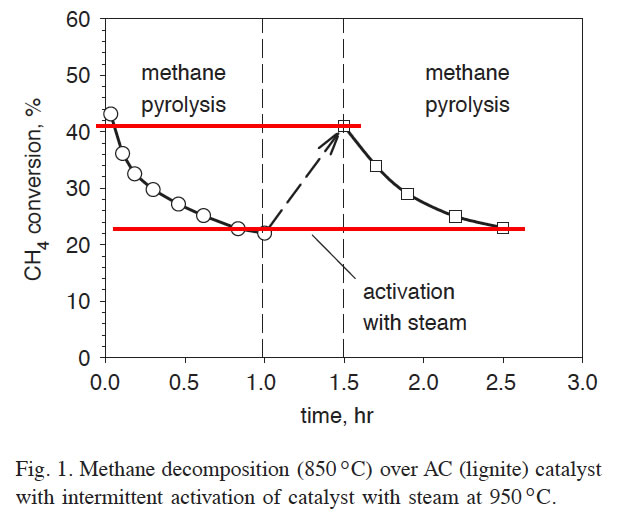
It is important to emphasize that although the regeneration of carbon catalysts requires the gasification of a certain portion of carbon (approximately, 15–20% of the total), the amount of CO2 produced (per unit of H2) would be a fraction of that generated during a metal catalyst regeneration (where all carbon has to be combusted or gasified). Estimated CO2 emissions from TCD process would be significantly less than those from SMR process. This is shown below.
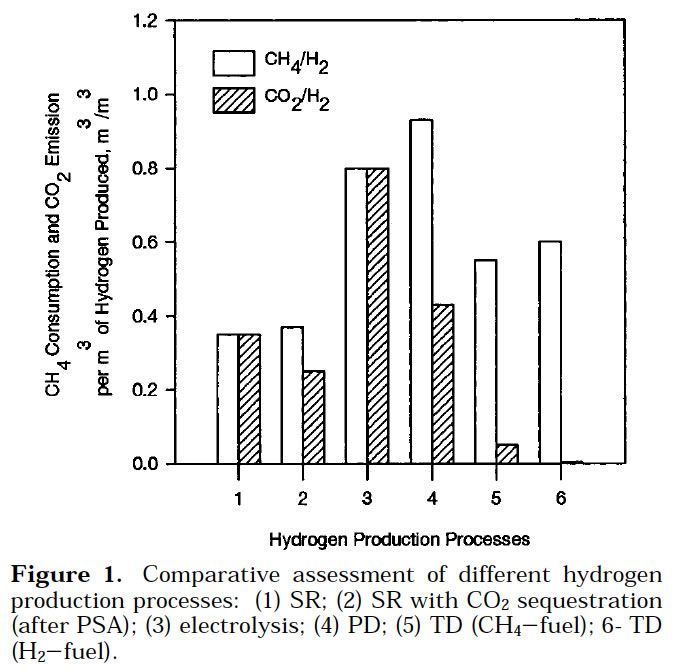
Note that processes 5 and 6, which we are discussing (we call then TCD/TD above) emit significantly less CO2 per unit of H2 than all others. In the figure above SR <=> SMR; PD <=> plasmochemical decomposition. Muradov [7].
One surprising result is that the processes with a large consumption of electric energy (water electrolysis, PD of NG) are characterized by the highest NG consumption and CO2 emission per unit of hydrogen produced. It should be noted, however, that this conclusion is based on the world average energy production scenario. Therefore, in countries with a large non fossil fuel energy sector (hydroelectric, nuclear energy) both E1(NG/H2) and E2 (CO2/H2) parameters could be much lower.
2.1.2. Costs
1998 estimates, from Muradov, while old are still informative.
TCD of NG is a technologically simple one-step process without energy and material intensive gas separation stages, while SMR is a multistep and complex process. The technoeconomic assessment showed that the cost of hydrogen produced by TCD of NG ($58/1000 m3 H2, with carbon credit) is somewhat lower than that for the SMR process ($67/1000 m3 H2).
Currently, the total world production of carbon black is close to 6 million tons per year, with prices varying in the range of hundreds to thousands of dollars per ton, depending on the carbon quality. Carbon black has a great market potential both in traditional (rubber industry, plastics, inks, etc.) and in new areas. For example, authors (8) identified the metallurgical industry as a very promising market for carbon black. Carbon black is particularly valuable as a reducing reagent for the production of SiC and as a carbon additive/carburizer in steel industry.
More cominng...
References
[1] Muradov N. How to produce hydrogen from fossil fuels without CO2 emission. Int J Hydrogen Energy 1993;18: 211–5.
[2] Steinberg M. Fossil fuel decarbonization technology for mitigating global warming. Int J Hydrogen Energy 1999;24:771–7.
[3] Gaudernack B, Lynum S. Hydrogen from natural gas without release of CO2 to the atmosphere. In: Proceedings of the 11th world hydrogen energy conference. Stuttgart, Germany, 1996. p. 511–23.
[4] Calahan M. Catalytic pyrolysis of methane and other hydrocarbons. Proceedings of conference on power sources, vol. 26, 1974, p. 181.
[5] Derbyshire F, Trimm D. Kinetics of the deposition of pyrolytic carbon on nickel. Carbon 1975;13:189–92.
[6] Robertson S. Carbon formation from methane pyrolysis over some transition metal surfaces-II. Manner of carbon and graphite formation. Carbon 1972;10:221–9.
[7] Muradov N. CO2-free production of hydrogen by catalytic pyrolysis of hydrocarbons. Energy Fuels 1998;12:41.
[8] Muradov N. Catalysis of methane decomposition over elemental carbon. Catal Commun 2001;2:89–94.
[9] Moliner R, Suelves I, Lazaro M, Moreno O. Thermocatalytic decomposition of methane over activated carbons: influence of textural properties and surface chemistry. Int J Hydrogen Energy 2005;30:293.
[10] Muradov N, Smith F, Raissi A. Catalytic properties of carbons for methane decomposition reaction. Catal Today, in press.
[11] Pohlenz J, Scott N. Method for hydrogen production by catalytic decomposition of a gaseous hydrocarbon stream. U.S. Patent No 3,284,161 (UOP); 1966.
[12] Kim M, Lee E, Jun J, Kong S, Hun G, Lee B, Lee T, Yoon K. Hydrogen production by catalytic decomposition of methane over activated carbons: kinetic study. Int J Hydrogen Energy 2004;29:187.
[13] Muradov N, Smith F. In: Marini M, Spazzafumo G, editors. Hydrogen power theoretical and engineering solutions. Padova, Italy: SGEditoriali; 2003. p. 87–95.
[14] Muradov N, Veziroglu T: From hydrocarbo to hydrogen - carbon to hydrogen economy. Int J Hydrogen 2005;30:225.
.