Methanol Fuels
1. Why add methanol to our fuel mixture?
Methanol can be made from natural gas, from coal, from any organic wastes, from algae, etc. It can also be made from hydrogen and carbon dioxide. Thus, it can be made, and is made, now, from both natural gas and from coal. It is also being made in the laboratory from H2 and CO2 -- i.e., totally renewably and total zero carbon. We will have to start using methanol from natural gas, but to eventually move it to its deserved renewable status, renewable methanol be recognized on an equal footing with second generation ethanol (cellulosic ethanol).
2. History of Methanol Use in Transportation
Methanol, which was originally made from the destructive distillation of wood, is now made in very large quantities in plants using natural gas as the feedstock. We will examine its role in transportation, and its costs relative to petroleum products gasoline and diesel, both of which it can replace as fuel for internal combustion engines. And we will look at methanol as a fuel for Fuel Cells.
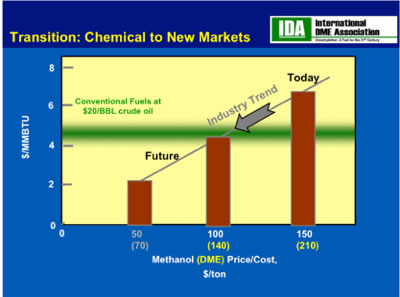
The chart below from the International Dimethyl Ether Association (IDME), which must make methanol and dry it to make DME, projects the cost of methanol to decrease from $150/ton today to $50/ton in a decade. The fuzzy green line show where these costs fall with respect to crude oil at $20/barrel. Clearly, today with oil in the 90 dollar a barrel range, methanol is very competitive, but even more important methanol will become even more competitive as its production costs decrease and the price of oil continues to increase.
2.1. The California Methanol Fuel Experiment
This very successful experiment, on our own soil, gets it own page. Link to California methanol fuel experience.
2.2. Chinese Methanol usage
Roughly 8% of the fuel used in the Chinese transportation system is methanol. The Chinese are now the largest producers of methanol in the world. Although little hard facts are emerging, we will present performance and usage details as we get them.
2.3. Methanol as a fuel for fuel cells
Two types of Fuel Cells can use methanol as their fuel. The DMFC and the PEMFC. The DMFC, or Direct Methanol Fuel Cell is used in power backup and small material handling equipment, and so far has been developed to output up to 300 W very efficiently. For greater power the PEM Fuel Cell (PEMFC) which uses hydrogen as a fuel is the choice. Many vehicle manufacturers have experimented with these, but the cost of hydrogen, its distribution, and its need for high pressure storage have made their use impractical for motor vehicles. However, methanol (which is a very efficient hydrogen carrier) can be reformed onboard to provide hydrogen, and so is a much easier to use fuel for PEMFCs. It was experimented with more than 10 years ago, but then the concept of green methanol was not developed, and hydrogen appeared to be the greener fuel, so methanol was abandoned first, and later hydrogen was abandoned.

the QBEAK on display at the Group Exhibit Hydrogen + Fuel Cells in Hannover, April 2013 (Fuel Cell Today)
But methanol is coming back strongly again as the fuel for PEMFCs. Both Low Temperature and High Temperature PEMFCs have been developed. The Danish company Serenergy was established in 2006 as a specialist designer and manufacturer of high-temperature PEMFC integrated with methanol reforming. Methanol was its fuel of choice from the beginning and the first question to address is why it decided on the HT-PEMFC + reformer route rather than DMFC. At power outputs above about 300 W PEMFC-based products make better sense as they allow for more compactness and efficiency than DMFC equivalents. Serenergy’s concept is also very specific in its ambition: as a serial range extender for hybrid vehicles where the battery caters to peak load and the fuel cell is sized to the average load. So rather than a power output of 80–100 kW, as is typical in FCEV, the fuel cell is only 5–20 kW so stack density can be lower (reducing cost per kilowatt but without increasing the system to an unmanageable size). The concept would work very well in a Volt type of powertrain, replacing the internal combustion engine.
Serenergy is in the planning phase with Denmark’s largest fuel distributor (OK a.m.b.a.) to adapt existing filling stations to dispense the cheap methanol-water mix used by QBEAK (the distributor is already delivering the methanol fuel being used in Material Handling Vehicle (MHV) trials in Denmark - like fork lifts). Telecommunications backup power is also a focus point: Serenergy's Jensen says the Serenergy product should be cost-competitive with a smaller footprint than LT-PEMFC + reformer products and will be designed as a straight replacement for diesel generators. Echoing the trend in Ballard’s sales (the Canadian Fuel Cell maker) of methanol FC over hydrogen FC, he believes methanol-fueled systems offer a superior alternative in many cases where longer runtimes are required. HT-PEMFC tolerate impurities in reformed fuel better than low-temperature PEMFC and the waste heat can be reused in the reforming process, allowing for simpler balance of plant, but HT-PEMFC technology has been plagued by reports of poor lifetimes. Jensen says this is in the past: the company’s MEA supplier has been delivering consistent product quality at volume for some time now and Serenergy has demonstrated 5,000 operating hours at the system level, allowing it to offer a warranty to that effect – although Jensen says the technology is expected to have a lifetime of 20,000+ hours as 30,000 hours has been demonstrated at the MEA level.
But what about carbon dioxide emissions? A vehicle fueled with methanol will emit CO2 at the tailpipe but in Denmark, where the aim is to use domestic energy resources as efficiently as possible, this may not be the deciding factor. Jensen points to a Danish study that indicates, under the stated conditions, well-to-wheel fossil CO2 emissions can be lower than hydrogen because methanol can be derived from a variety of renewable resources with good yield and high efficiency and because it is relatively easy to store and distribute. And, even more CO2 neutral, renewable methanol is becoming available?
3. Production of Methanol
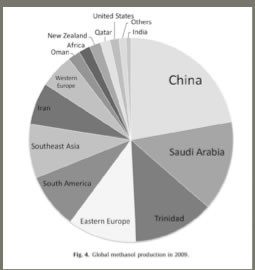
Worldwide, China, Saudi Arabia, Trinidad and Eastern Europe lead in the production of methanol, and there is a gradually decreasing amount in ten remaining countries, with the US near the bottom of the list.
3.1. History of US Methanol Production
Methanol was initially made in the US over 100 years ago from the destructive distillation of wood, and hence was called wood alcohol.
3.2. Emissions from Methanol Production From Natural Gas and Coal, CO2 and Hydrogen
A very large amount of methanol is made world wide. The Methanol Institute is a very good source of information on these statistics and many other aspect of methanol. The most common way today is to use the gas phase methanol reactor. This technique uses natural gas to make methanol. However, in China methanol is made using coal, as it was in the US in the early part of the 20th century. It is made this way in the US in Kingsport Tennessee. Another method that has gained little attention is the liquid phase methanol process. We describe these below. The plot shows two other ways as well: using flue gas and hydrogen and obtaining the CO2 from the atmosphere and using hydrogen with it. The value of 1 on this chart (red line) is the amount of GHG resulting from gasoline.
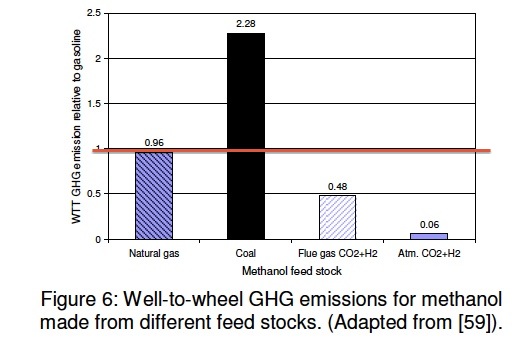
The coal-to-methanol carbon intensity value in the figure above assumes that the plants only make dedicated methanol. Plants that combine methanol production with electricity generation have the potential to produce methanol more efficiently than stand-alone plants. The efficiency of producing methanol using the Air Products [ARCADIS] methanol production technology has been estimated to be 71% (see liquid phase methanol technique below), compared with the 55% assumed here for stand-alone plants [Fletcher]. Methanol produced in these facilities or in PolyGeneration units in China would have correspondingly lower GHGs
3.2.1. Gas phase methanol production
The most common way to make methanol is to use natural gas in a steam reformer, to make a synthesis gas of H2 and CO and then pass this snygas over a catalysts.
3.2.1.1 Natural gas
Natural gas is the most common feedstock for modern methanol production outside of China, which uses coal.
3.2.1.2. Using waste CO2 and Wind generated Hydrogen
Methanol can be made entirely 'green' buy using H2 (from wind turbine powered electrolysers) and CO2 captured from the air. Although there are startups pursuing this approach, such as Air Fuel Systems, no commercial venture have energed as yet.
3.2.1.3. Using geothermal energy and CO2
3.2.2. Cost reduction of gas phase methanol with increasing size of the plant.
Note that at a petroleum fuels cost of $20/bbl the curent cost of methanol would be 'break-even'. It is however forecast that the cost of methanol will decrease further to $2/mmbtu (i.e., $50/ton) in the future.

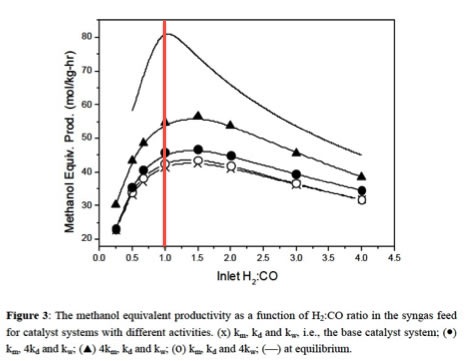
3.2.3. Methanol Productivity as a function of the composition of the sygas created
Different feedstocks produce different ratios of hydrogen to carbon dioxide (H2/CO). Furthermore, different methanol production processes work best in different ranges of H2/CO.
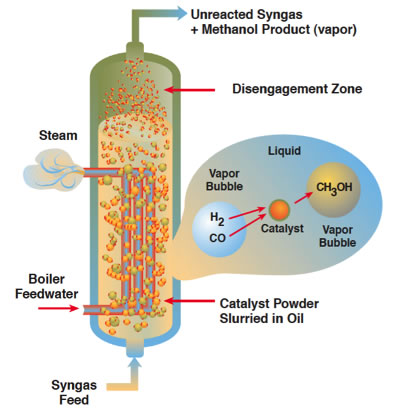
3.3. Liquid Phase Methanol Technique
This methanol production technique uses very fine particles of catalysts suspended in a tank of oil (like mineral oil) that is heated and pressurized. From the bottom, syngas, a mixture of of hydrogen and carbon monoxide, is bubbled through. The syngas can be created from practically any source: natural gas, coal or biomass. The reaction with the catalysts produces methanol and heat. The methanol comes out the top, and because water is used to cool the reactor, it is converted to steam, which can be used to run a steam generator, heat other parts of the plant, or as we will see later, assist the fermentation process in an ethanol plant.
3.4. Methanol from Organic wastes (Corn husks, mill wastes, food wastes, animal manure, etc.)
Very much in a way that we think about how good cellulosic ethanol would be if we could make it ===economically, making methanol from organic wastes is a real economic proposition now!
It can be made in at least two different ways. One is to anaerobically digest the wastes to create biogas, and then put the resulting methane into a standard gas phase methanol plant. A second way, more than a hundred years old is via the destructive distillation of word
3.5. Methanol Production Can be CO2 Neutral
Methanol production from organic wastes has the potential to be CO2 neutral. There are a number of methanol production plants doing this now. The largest operation is in Sweden where paper mill waste called black liquor is converted to methanol. More coming.
3.6. Dry Natural Gas Reforming
Methanol can be made from a wide variety of syngas. Syngas that is created by the reforming of natural gas and carbon dioxide at temperatures greater than 1100C without the use of either water or catalysts is one example. This 'dry reforming' of natural gas produces a ration of H2 to CO of 1. This ratio is more effective in the liquid phase methanol process. More to come.
3.7. Methanol from Hydrogen and Carbon Dioxide
The production of methanol using hydrogen and carbon dioxide has appeared to be an expensive proposition, largely because of the costs of decomposing water by electrolysis to obtain hydrogen.
3.7.1 Catalytic Methane Decomposition
An alternative way to obtain the hydrogen is by Catalytic Methane Decomposition (CMD). CMD can supply the needed hydrogen for much lower costs. If there is a source of CO2, then methanol can be made from the H2 and CO2. We discuss the pros and cons of Catalytic Methane Decomposition here.
3.8. Future Methanol Production Techniques using solar energy
3.8.1. Multielectron Reduction
Methanol can be made directly a process called "Multielectron Reduction of CO2". The electrochemical reduction of carbon dioxide (CO2) has generated substantial interest over the past decade as a way to potentially convert this greenhouse gas into useful fuels and small organics. More coming...
3.8.2. Syngas via concentrated solar energy
Using either dry reforming or catalytic assisted dry reforming and concentrated solar energy appears to be a very desirable way to make syngas and then methanol. Of course the methanol can be used as a precursor for gasoline (the Mobil MTG process) or other fuels via Fischer Tropsch process.
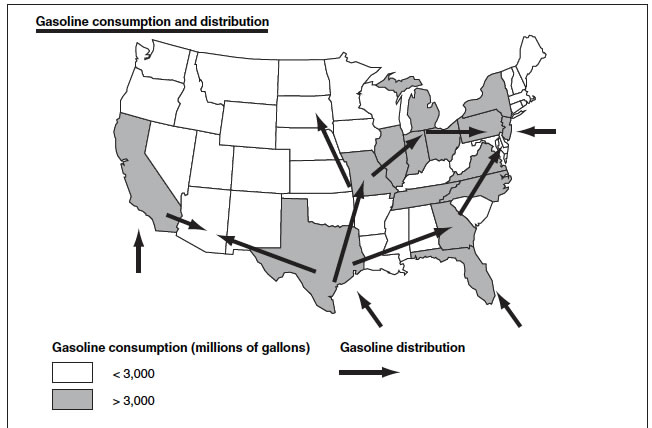
4. Methanol Distribution will be easier than ethanol distribution and even easier than gasoline distribution.
See maps below.Gasoline distribution.
Ethanol distribution: essential from the mid-west, and Overseas.
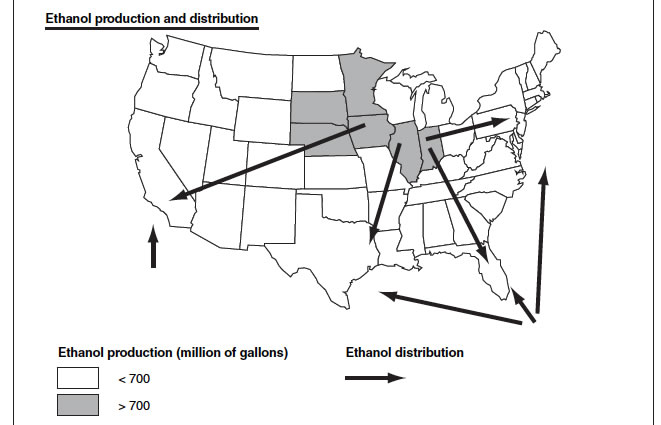
Potential methanol plants and production can be distributed all over the US as they can be located near to the Natural Gas deposits.
5. Methanol Fuel Efficiency and Emissions
5.1. The most often voiced objection to methanol fuel is stated in the wrong terms
This is the low energy content of the fuel in a gallon compared to that of gasoline, which relates to lower range in currently tuned engines get the most attention. As the table shows, methanol has 16 MJ/l compared to gasoline with 34 MJ/l.
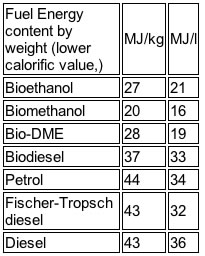
However, there is more to the story. The major additional asset methanol has is its high octane number (110). Its octane number is so high that we can get just as far on a gallon even though the energy content is only half that of petrol (see above).
Methanol can with current automotive engines easily replace 30% of gasoline and it is easy to make a motor for pure methanol, according to the Danish Methanol Association. We will investigate this further, when we review the work of the EPA, Saab and Lotus.
But first, note that both the US EPA and Saab Motors showed that the higher thermodynamic efficiency that an engine is able to run at enables it to compensate for its lower energy per unit volume.
An extensive look at the situation actually shows the remarkable value of methanol. If we think of the energy needed to go a mile, methanol clearly outperforms gasoline (petrol, as the Europeans say) and even outperforms ethanol (data from the Danish Methanol Society).
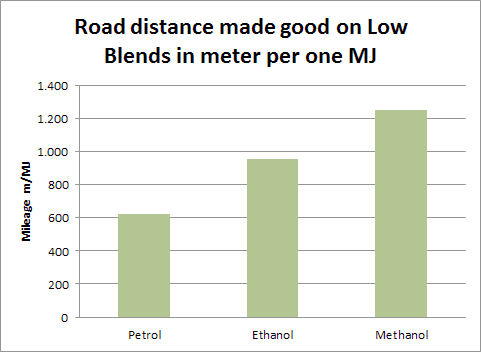
So, if we are thinking energy usage, as the Institute for Energy Resourcefulness is focused on, using less of the energy in the feedstocks that we can make the fuels out of, is the preferred way to go.
5.2. Methanol at its performance best - our future.
“…The impact on the temperature decrease by alcohol fuels is significantly higher than for petrol. In the ethanol case, it is a reduction of 138 K [Kelvin] and for the methanol even higher (246 K). This improves the anti-knock properties substantially to the level of equivalent to an octane number of 129 in the ethanol case [and Research Octane Number 133 for methanol]. If this feature was utilized on a turbocharged engine to increase power density and further on for downsizing of the engine to reach the baseline power and performance, the two reports cited have calculated that the energy consumption could be reduced by some 25 % compared to a conventional petrol engine. The gain of 25 % is roughly equivalent to the relative difference between petrol and diesel engines in such vehicles. Bearing in mind that the average (relative) increase in efficiency of the engine has been about 0,5 % per year over the last 75 years or so, an improvement of this magnitude would be monumental.”
“Brusstar and co-workers conducted a study of great interest for future engine development of alcohol-fueled engines (Brusstar et al., 2002). A 1,9 liter VW TDI diesel engine was used in these experiments. Engine modifications included port fuel injection and spark ignition. Cooled EGR [Exhaust Gas Recirculation] using a low-pressure long route (after turbine to before compressor) EGR system was used instead of the standard EGR system. Compression ratios between 17:1 and 22:1 were tested but the nominal compression ratio of 19,5:1 was used during most of the test series.”
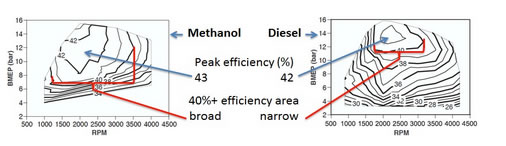
“The methanol-fueled engine had a peak efficiency of nearly 43 %, i.e. higher than the diesel-fueled baseline engine, and the high-efficiency range was also broader than in the diesel case. Fueled by ethanol, the engine had comparable peak efficiency to the diesel-fueled engine. The knock sensitivity when operating on ethanol prevented the engine from achieving as high efficiency as when it was run on methanol.”
In the figure below the efficiency maps of the engine running on methanol and diesel is seen. BMEP stands for Brake Mean Effective Pressure and RPM stands for Revolutions Per Minute. The brake efficiency exceeded 40% over a power range of 12 to 75 kW for the methanol fueled engine.
(An exhaust characterization study based on regulated and unregulated tailpipe and evaporative emissions from bi-fuel and flex-fuel light-duty passenger cars fueled by petrol (E5), bioethanol (E70, E85) and biogas tested at ambient temperatures of +22°C and – 7°C, Westerholm, Ahlvik & Karlsson, March 2008, p. 14-16).
So we could replace both gasoline and diesel fuel using methanol.
5.3. Methanol emissions produce less ozone
Ozone, or photochemical smog is formed in the lower atmosphere when sunlight reacts with hydrocarbons (HC) and nitrogen oxide. It is generally regarded as the most serious non-attainment problem. The vehicle contribution to hydrocarbons comes from two sources: the tailpipe and evaporative emissions. One of the approaches to lowering the motor vehicle contribution to ozone formation is to change the character of the tailpipe emissions. This is one of the ways in which methanol vehicles make a contribution to improvement in air quality. It is not the level of the HC tailpipe emission per se that is lower; it is the composition of the emission that is different. Hydrocarbon compounds have varying levels of reactivity in the atmosphere, as shown in Table 2 below. Fuels that produce less reactive exhaust emissions when burned, will generate less ozone. As can be seen, methanol is quite low on the reactivity scale compared to some of the more complex hydrocarbons associated with gasoline. The ozone reduction associated with the use of methanol is also dependent on proper control of the formaldehyde emission. Fortunately the technologies that control HC emissions, in general, are the same kind of controls that control formaldehyde; in both cases, almost all of the emission is produced in the first two minutes of operation after a cold start 3. Beginning in May 1993, the California Air Resources Board (CARB) included an emission standard for formaldehyde of 0.15 mg/min, which was approximately the formaldehyde emission level of the typical gasoline vehicle. The emission standard also included a reactivity factor for the various alternative fuels. For methanol, it was about half that of gasoline.
The methanol vehicle evaporative emission benefit is difficult to define. If the vehicle is dedicated to operation on methanol, the evaporative HC emission is definitely lower (and less reactive) because of the low vapor pressure of methanol. But, for the FFV, the vapor pressure can vary greatly since the mixture in the tank can vary from all gasoline to all methanol, and anything in between. When the 3.0L FFV Taurus went to production in 1993, four canisters were required to certify them for evaporative emission control across the full spectrum of fuel combinations.
Table 2- Photochemical reactivity of organic compounds:
Rate constants for reaction with hydroxyl (OH) radical
| Compound | K-4 x 10-1 (ppm/min) |
| Trans-2-butene | 10.5 |
| 1,2,4 Trimethyl benzene | 4.9 |
| M-xylene | 3.4 |
| Propionaldehyde | 2.2 |
| Acetaldehyde | 2.2 |
| Propene | 2.1 |
| Formaldehyde | 2.1 |
| Ethylene | 0.45 |
| N-butane | 0.35 |
| Propane | 0.25 |
| Methanol | 0.148 |
| Ethane | 0.045 |
| Acetylene | 0.022 |
| Carbon monoxide | 0.0012 |
5.4. A caution when burning methanol
When methanol is burned, formaldehyde is one of its products of combustion. First, the quickly stated bad and good of it, and then we will delve into detail. Formaldehyde has been found to be carcinogenic, resulting in cancer of the ...... However, the current 3-way catalysts in our exhaust systems can capture the formaldehyde. The concentrations are low initially, and in the California Methanol Experiment were considered acceptable. More later...
6. Other issues with methanol that have Engineering Solutions
6.1. Pure Methanol has cold starting problems
The vapor pressure of pure methanol (M100) is 4.6 psi. If we mix a little gasoline (15%) to get M85 the vapor pressure increases to 7 psi, making cold starts possible in most climates. There, of course, may be many other ways to avoid the cold start problem. For example, in cold weather regions, the user generally relies on a block heater even with gasoline.
6.2. Pure Methanol burns invisibly in daylight
Without the complex hydrocarbons of he gasoline, the flame of methanol combustion is invisible in daylight, which was a concern in the case of a vehicle fire. M85 solved that problem also. But, once again it is not the only way to solve this potential problem.
6.3. Pure Methanol has a very low vapor pressure, so the vapor in a tank can possibly ignite.
The other issue was the flammability limits of MIOO. At normal ambient temperatures, the air-fuel mixture ratio of the gasoline vapor above the liquid in the tank is too rich to ignite. This is not true for the alcohols because of their low vapor pressures. The addition of 15 per cent gasoline moves the flammability limit temperatures to a more acceptable range of risk, close to that of the gasoline vehicle. Once again, these problems have been resolved.
6.4 Health Impacts
A. Methanol is produced in small amounts in the human body as part of the metabolic process, and occurs naturally in some fruits. At high enough concentrations it is poisonous. Ingestion of 10 ml can cause blindness and 60-100 ml can be fatal if the condition is untreated. [Toxicity]
US OSHA permissible exposure limit for general industry in air (40 h/week) is 1900 mg/m³ for ethanol, 900 mg/m³ for gasoline, and 260 mg/m³ for methanol. When methanol is blended in gasoline, although the overall vapor pressure increases (see section VI), the partial vapor pressures of some gasoline compounds with high toxicity, such as benzene and 1,3 butadiene, decreases. It is difficult to smell methanol in air at methanol concentrations less than 2,000 ppm (1500 mg/m 3 ). Figure 10. Oral LD50 values for common fuels [Short Stark] For exposure to toxic chemicals, the most common measurement of prompt toxicity is the median lethal dose, LD50, defined as the dose at which 50% of the test population is killed. For methanol there are three primary methods of exposure: ingestion, inhalation and dermal contact. [Stark] The toxicity of chemicals is determined by observing following exposure the response of a population of test animals (commonly mice and rats, but in some cases, rabbits and dogs), compared with a similar population that is not exposed. The duration of exposure and the time of 52 observation are not uniform across investigators, but there are well known rules to adjust for variations.
For exposure to toxic chemicals, the most common measurement of prompt toxicity is the median lethal dose, LD50, defined as the dose at which 50% of the test population is killed. For methanol there are three primary methods of exposure: ingestion, inhalation and dermal contact. The LD50 health effects of conventional fuels as well as alcohol fuels are shown in Figures 10. 11 and 12 for oral, inhalation and dermal contact, respectively.
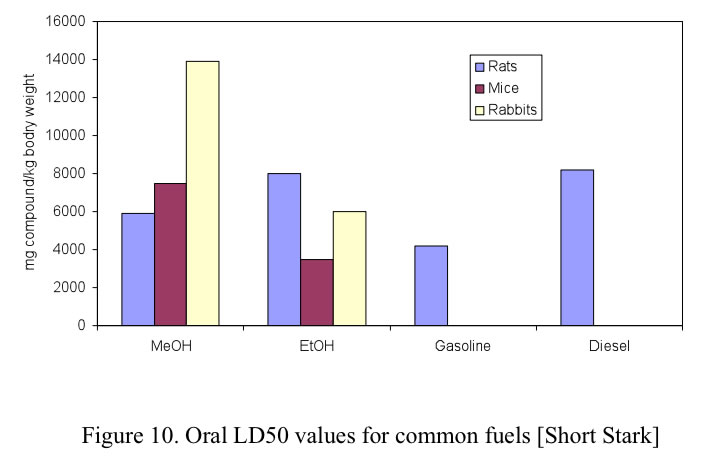
These figures show that methanol toxicity resulting in death as a result of ingestion, is comparable in terms of LD50 to the other fuels.
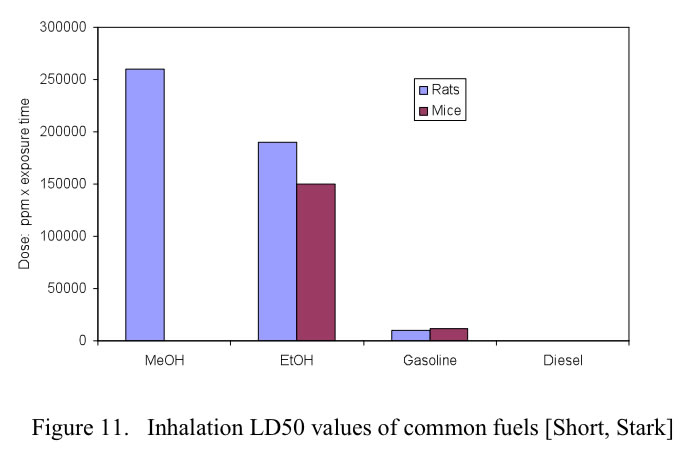
In the case of inhalation of the vapors, methanol is actually less toxic than either gasoline or ethanol. The plot shows that it takes more methanol to reach the LD50 level (adapted from Stark, Short)
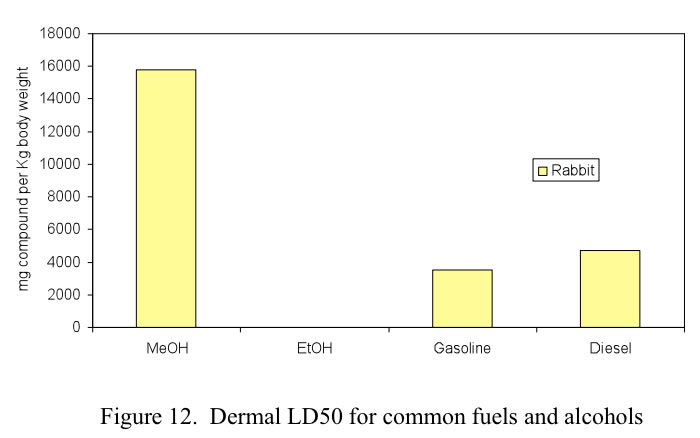
Dermal absorption during accidental exposure during refueling is a concern. Methanol and ethanol are either even or less toxic than gasoline or diesel. Again the graph shows that it requires much more methanol on the skin to achieve the LD50 level. Comparison of risks of alcohol fuels to those of fossil fuels is shown in Figure 12.
The use of methanol as a fuel has met resistance, more than most fuels, due to claims that it is unsafe for public utilization due to toxicological concerns. As discussed above, the issue of toxicity is often overstated as methanol’s toxicity (death) is on the same order as other fuels being considered as gasoline and diesel substitutes. Further, methanol is being used and has been widely used as windshield washer fluid without any major concern, and in this case the fluid is literally spread across the road. In the California test, with over 200 million miles of methanol driving, there was not a single case of accidental methanol poisoning.
The availability of ethanol and fomepizole as antidotes has dramatically enhanced the ability to treat these overdoses and subsequently reduce related toxicities. [McMahon]
7. Why we will need and want to go the use of methanol and methanol/ethanol/gasoline blends
1. We can not make enough ethanol from corn to meet the current national requirement. See the Ethanol Blend Wall in our Ethanol Fuels Production Section.
2. We may be decades away from making enough cellulosic ethanol - discussed in Ethanol Fuels Production Section.
3. We have the natural gas resources needed to make methanol. See our Domestic Natural Gas and our Renewable Energy/Bio-Fuels pages.
4. We avoid the three issues discussed in Sections 6.1-6.3, above.
5. Methanol blended in with gasoline (E10 or E15) will cost less to drive on. This plot from Lotus, shows that if we use GEM blends (which keeps the power output and derivability of an E85 Flex Fuel Vehicle the same), the cost of the Blend will go down as the amount of methanol in it increases.
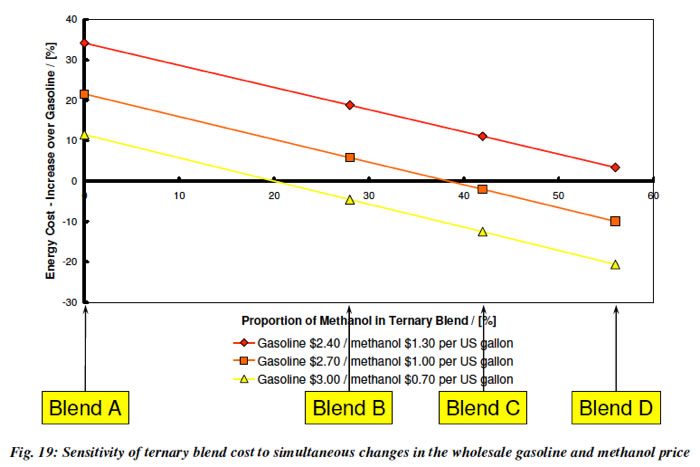
Turner et al. 2011 GEM Ternary Blends: Removing the Biomass Limit. SAE 2011-24-0113
This plot is brought into reality, by a famous experiment performed by Robert Zubrin with his 2007 Chevy Cobalt (that we link to here). Zubrin ran pure methanol and compared it to E10, so this is an extreme case, which shows more savings than above, but is interesting because without any changes in his vehicle in the form of tuning, or timing, or whatever, but just by letting the on-board computer sort the differences, remarkable differences are observed. As we have noted, if changes are made, i.e., starting with a Flex Fueled car for example, even more savings would have been achieved.
As a result of Zubrin's experiment we could calculate the difference in costs if he drove his Cobalt 36,000 miles, say, on either methanol M85 or gasoline (E10).
According to Matt Roberts Government Affairs - Americas/Europe Methanol Institute, here is how the numbers should be obtained. Methanol costs $1.12 a gallon at the time of the calculation (4/2/12) for the Gulf Coast spot price. You would add $0.46 for taxes, retail, distribution and markup. This number was developed by TIAX in a study of methanol fuel. So methanol (M100) at the pump would retail for about $1.58. The more likely blend we would see at the pump though would be M85 (15% gasoline) – this would cost about $1.90 with all taxes and markup included (raw 83 octane gasoline is at $3.25 a gallon right now).
Methanol contains about half of the heating value (BTUs) of raw gasoline, a touch more than half of the BTUs of reformulated gas with ethanol. Now if you don’t take advantage of the higher octane of methanol, and burn it in a standard vehicle set up – this means you will get less mileage per gallon. To factor this in, we do what is called a Gasoline Gallon Equivalent (GGE) cost, and do a straight ratio of energy.
So M85 fuel ($1.90 at the pump) would have a GGE cost of $3.25. AAA Fuel Gauge has regular gasoline at $3.93 today, and E85 (adjusted for GGE) selling for $4.43. This means you would save about $0.68 a GGE on M85.
Based on these inputs defined above - the spread over 36,000 miles would be more like $4415 for gasoline and about $2,600 for methanol, depending on the actual performance of the Cobalt. That is roughly half the cost — and all of the profits and associated revenues would remain in America. And we would have the added benefit of cleaner air.
8. What about the number of methanol capable pumps needed?
In the early days the question of how many methanol stations would we require before significant use of methanol and sufficient vehicles would be purchased was of primary concern. However, now with the ability to use any combination of ethanol, methanol or gasoline, there is time to have filling stations evolve. It is still interesting what the number that was arrived at was. Nichols summarized this picture: "At the time, about five per cent of the refueling stations had diesel fuel, primarily because of the truck stops. This appeared to be sufficient for diesel passenger cars to be bought by the retail consumer. Since a methanol-fueled vehicle would require more frequent refueling because of its lower energy density per gallon, it was estimated by one major oil company that 10 per cent of the refueling stations would need a methanol pump for introduction of the dedicated methanol vehicle. In 1980 the cost of this was estimated to be about $600 million and when these facilities were first in place, there was lot of "real estate" devoted to methanol pumps and storage tanks with very little activity since it would take some time for methanol vehicle populations to grow."
But all this is a thing of the past. The drive for filling station owners to install methanol pumps will be to attract customers; because they could offer fuel at a lower price. Furthermore, off-the-shelf Blender pumps (see Ethanol/Value of .....) would not cost significantly more than gasoline pumps - and since they are capable of using any fuel, the risk to a station owner would be minimal.
References
[Fletcher] J. Fletcher, V. P. Roan and D. Betts, An Investigation of the Feasibility of Coal-Based Methanol for Application in Transportation Fuel Cell Systems presented at the 3rd International Energy Conversion Engineering Conference, San Francisco, California, AIAA 2005-5606 (August 2005)
[McMahon] D.M. McMahon, S. Winstead and K.A. Weant, Toxic Alcohol Ingestions: Focus on Ethylene Glycol and Methanol, Advanced Emergency Nursing Journal 31, No. 3, pp. 206–213 (2009)
[Short] G. D. Short, Fuel methanol. In Methanol production and use, W. H. Cheng, H. H. Kung, eds. New York: Marcel Dekker, Inc 1994
[Stark] Stark, A.K., Multi-Criteria Lifecycle Evaluation of Transportation Fuels Derived from Biomass Gasification, Master of Science in Technology and Policy and Master of Science in Mechanical Engineering, MIT, February 2010
.